Our Technology
Limbal stem cell deficiency (LSCD) represents a significant ophthalmic condition that results in corneal blindness, affecting approximately 375,000 patients in Europe and nearly 4 million individuals on a global scale. A unique approach to address LSCD is to restore the healthy limbal stem cell population, which has become dysfunctional, within the eye. To treat this disease, cellular therapy aproach and products has been developed and proposed as a highly promising solution. Indeed, the global cellular therapy market size is expected to reach € 89 billion by 2033 with compound annual growth rate of 22.66% from 2024 to 2033.
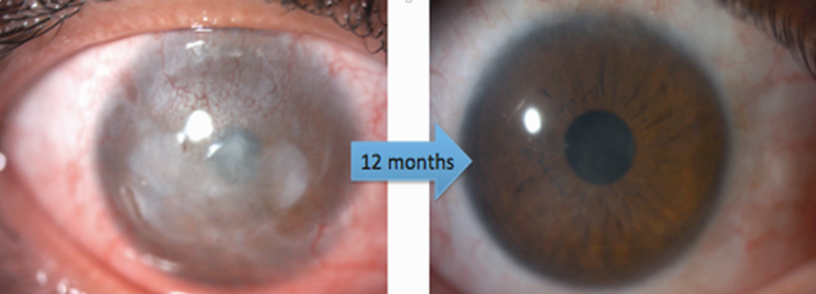
The founding team of Limbustem has enhanced the sophisticated technology of limbal epithelial cellular therapy. In this technology, a limbal biopsy measuring 1-2 mm is cultured on human amniotic membrane using human serum, thereby facilitating the expansion of therapeutic limbal cells in vitro. Subsequently, the in vitro-produced cells have been transplanted to the affected eye. Following the receipt of approval from the relevant health authorities, Limbustem has introduced this technology to the local market and has thus far delivered the product to 17 patients.
Our innovation is AME supplementation into the cell media of in vitro cultured limbal epithelial cellular therapy product (Limbustem PRO AMEX).
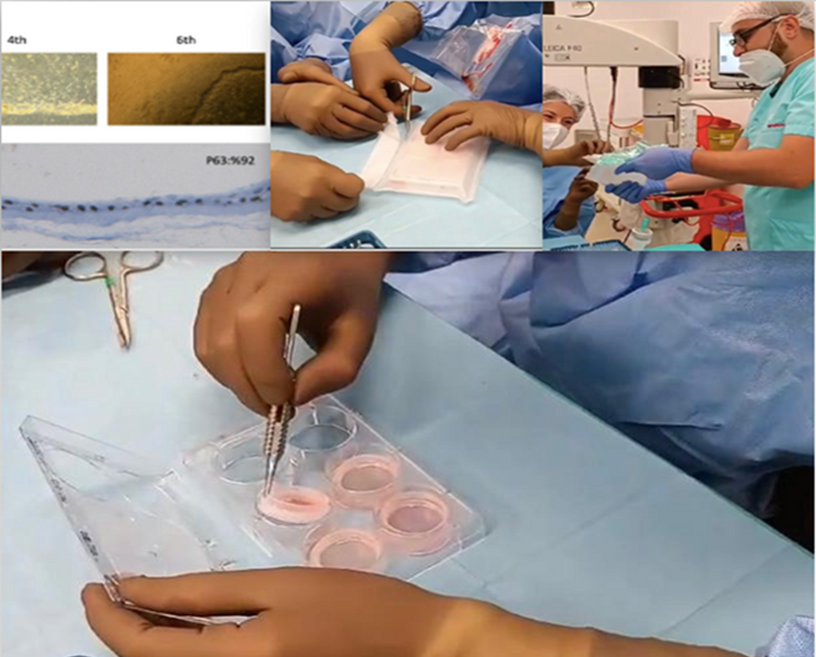
Limbustem is also engaged in the further development of the commercially available product, with the objective of enhancing the technology (e.g., increased stem cell ratio and clinical success). To this end, the team has put forth the proposal of utilizing human amniotic membrane extract (AME) as a culture supplement with the objective of enhancing the biomimicry of the in vitro culture environment. This is based on the concept that AME contains a plethora of bioactive molecules, including extracellular matrix proteins (e.g., collagens, laminin) and growth factors (e.g., EGF, NGF), which have been demonstrated to support the maintenance of limbal epithelial stem cells. The incorporation of amnion membrane extract into the in vitro environment of the cells represents a novel approach to this technology, resulting in an increase in the stem cell ratio from 50% to 80%. This is expected to result in a higher rate of clinical success.
